CALGARY -- Some Calgary parents are welcoming news from Pfizer stating its vaccine has been found to be effective in youth as young as 12 years old.
"I’m all for vaccinations," said Blaine Everingham, whose son Corbin will turn 12 this summer. "I think it protects society so I will fully support when he can get the vaccine as soon as possible."
If the vaccine is approved for use, the father and son would both support scheduling an appointment for Corbin.
"I want to not have to wear masks," said Corbin. "It’s just less fun at school when you have to wear masks and do COVID procedures and everything,"
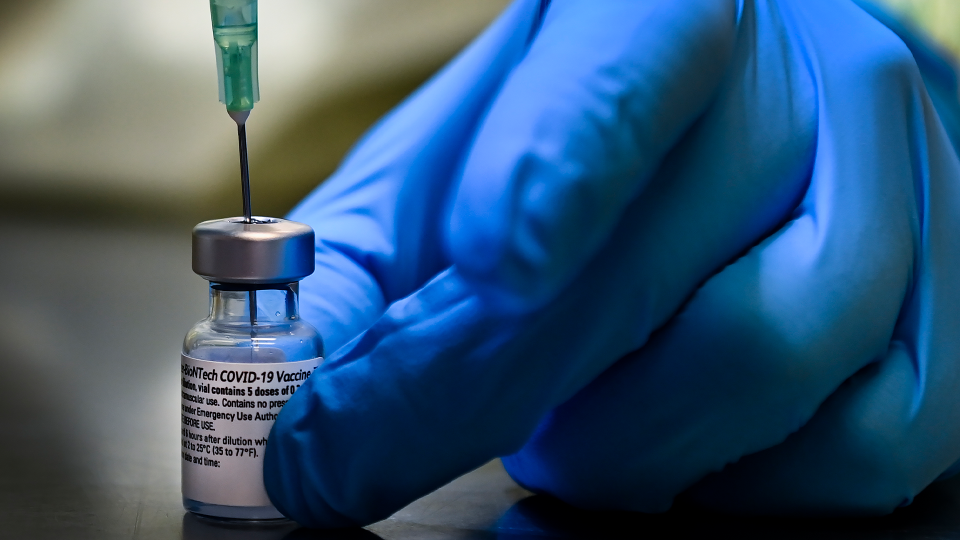
The drug maker announced Wednesday that its COVID-19 vaccine is safe and strongly protective in kids between the ages of 12 and 15.
"Our lives have changed almost completely in the last year because of this pandemic," said Phil Canji, who has children aged 10 and 12, "so I’m all for getting my kids vaccinated and trying to get back to some sort of normalcy."
The company released preliminary data showing 2,260 youth in the United States participated in a clinical trial and none of the teens who received the vaccine developed COVID-19.
The company said there were 18 participants who were given placebo shots.
The results will now be presented to the U.S. Food and Drug Administration for approval.
The Pfizer-BioTech vaccine has already been approved in Canada for people as young as 16, but there’s no timeline for when the younger age groups could potentially get a shot.
"With any of these changes in terms of how the vaccines are used, the companies have to come to Health Canada, give us the data," said Dr. Supriya Sharma, chief medical advisor with Health Canada. "We review it to make sure that the vaccines are safe and effective and of high quality and that the benefits outweigh the risks in whatever population that it's studied in."
Sharma said clinical trials are typically done in adults first, but clinical trials in children can be with smaller groups.
"They handle and they metabolize, and they respond to medications and vaccines in different ways so we have to do the trials in different age groups."
Sharma adds that other companies — including Moderna, AstaZeneca and Johnson & Johnson — are also conducting trials in children.
Alberta Health said it is following guidance from Health Canada.
“We will continue adjusting our approach based on the emerging evidence in the coming months,” said Tom McMillan, assistant director, communications, Alberta Health.
The province said Alberta’s vaccine rollout is prioritizing immunizing those at the highest risk of severe outcomes.
Alberta is planning to offer the vaccine to all Albertans age 18 and older by the end of June.
The exception is phase 2B which began Tuesday, which focuses on Albertans with underlying conditions that put them at high risk of sever outcomes. Now Albertans who are 16 and older are being included. Pfizer is currently licensed for use in this group.
All other vaccines in Canada that are currently approved are licensed for ages 18 and older.