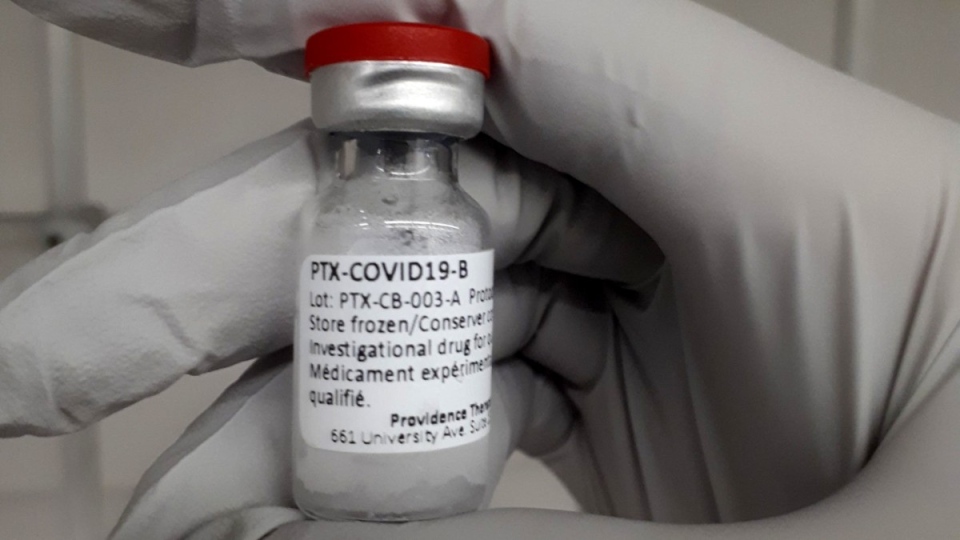
CALGARY -- Canadian vaccine maker Providence Therapeutics is hoping to gain more interest and funding from the federal government as it continues human trials on its coronavirus vaccine.
The company, which has offices in Toronto and Calgary, recently received a $5 million grant from the National Research Council of Canada, but the vast majority of the project has been privately funded.
Providence Therapeutics CEO Brad Sorenson says his company has already spent more than $10 million.
He notes that more funding is needed to expand capacity as Canada deals with vaccine shortages from both Moderna and Pfizer, along with the threat of vaccine export bans from the European Union.
“It’s important that we have strategic capacity and it’s not so much the question of vaccines we have now. It’s about looking forward and understanding that this isn’t going to go away this fall,” Sorenson said.
“We’re going to have variants and we need to have strategic capacity to respond to those variants within Canada and we will prioritize Canadians.”
Sorenson says he has had “no meaningful discussions” with Ottawa, but does have a meeting scheduled on Wednesday with Minister of Science and Industry Francois-Phillipe Champagne.
No production funding announced for Calgary-made vaccine
Pending approval from Health Canada, Prime Minister Justin Trudeau announced a tentative deal on Tuesday with American company, Novavax, to pump out millions of doses of coronavirus vaccines at a new National Research Council facility in Montreal.
Trudeau also touted the ability to produce vaccines in Saskatchewan or Vancouver, but what about Calgary?
Calgary manufacturing company Northern RNA is working alongside Providence Therapeutics to create some of the building blocks of messenger RNA for the vaccine.
Known as mRNA — this is what provides instructions to cells to build proteins that may treat or prevent diseases and has been validated as 95 per cent effective against the COVID-19 virus in both Pfizer and Moderna trials.
Northern RNA CEO Brad Stevens calls this a "game changer" for the Providence Therapeutics vaccines and pending Health Canada approval, he hopes the federal government will recognize the urgency to get millions of doses to the Canadian public.
“We could all slow play this a bit, but the most important part is actually timing and with some further funding we can be up in production by the end of the year,” Stevens said.
“This is an investment in jobs and in hard infrastructure and ultimately once that is built we can either triple or quadruple the number of jobs we would have and it’s desperately needed in Calgary and Alberta.”
Stevens adds that Northern RNA is ready to start production as soon as possible and has been contacted by multiple vaccine makers in Canada and around the world.